Individualized positive end-expiratory pressure guided by end-expiratory lung volume in early acute respiratory distress syndrome: study protocol for the multicenter, randomized IPERPEEP trial
Enrolments are open!
See rationale and details of the IPERPEEP protocol
Free full text here on Trial
Background
In acute respiratory distress syndrome (ARDS), response to positive end-expiratory pressure (PEEP) is variable according to different degrees of lung recruitability. The search for a tool to individualize PEEP based on patients’ individual response is warranted.
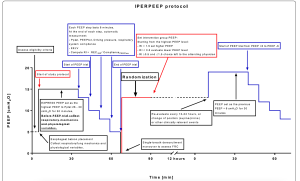
End-expiratory lung volume (EELV) assessment by nitrogen washin-washout aids bedside estimation of PEEP-induced alveolar recruitment and may therefore help titrate PEEP on patient’s individual recruitability.
We designed a randomized trial to test whether an individualized PEEP setting protocol driven by EELV measurement may improve a composite clinical outcome in patients with moderate-to-severe ARDS (IPERPEEP trial).